Ceramides may play a role in early-onset Parkinson’s disease
The researchers in the laboratory of Dr. Hugo Bellen, professor at Baylor College of Medicine and an investigator at the Howard Hughes Medical Institute and at the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital, have found ceramides, a family of lipid molecules found within cell membranes, play an important role in an early-onset form of Parkinson’s disease. The study was published in the journal Cell Metabolism.
The researchers propose ceramides as the linchpin that connect previously identified cellular defects and genes independently known to be associated with Parkinson’s disease. They suggest increased ceramide levels in the neuronal membranes, due to mutations in PLA2G6 gene, as a common cellular stressor that causes neurodegeneration in Parkinson’s disease. The findings from this study could result in novel strategies to prevent or treat Parkinson’s disease in the future.
In this study, Dr. Guang Lin, postdoctoral associate in the Bellen lab, and colleagues developed a fruit fly model by knocking out iPLA2-VIA, which is the fly equivalent of the human PLA2GA6 (PARK14) gene. They found the mutant flies had shorter lifespan than normal flies, had locomotion defects and presented with cellular characteristics similar to those observed in humans with mutations in PLA2G6 gene. Younger flies carrying this mutation were healthy but they started showing signs of neurodegeneration as they aged.
Previous studies have shown mutations in PLA2G6 gene cause neurodegenerative disorders and early onset Parkinsonism in humans. Mice lacking this gene also exhibited progressive motor defects. This gene codes for a phospholipase, an enzyme that modifies a type of fats called phospholipids that are the fundamental building blocks of neuronal membranes. However, it was not clear how loss of PLA2G6 function caused progressive neurodegeneration.
Here, the researchers have identified the molecular events that lead to progressive neurodegeneration in the absence of functional PLA2G6.
First, they measured the effects of this mutation on bang sensitivity and the visual system. Bang sensitivity refers to the response of the fruit flies to mechanical stress and measures the resilience of the nervous system to mechanical stress. While normal fruit flies recovered immediately from the impact of being repeatedly banged, flies lacking the iPLA2-VIA gene took a long time to recover. It appears this relatively mild mechanical stress temporarily paralyzed the mutant flies indicating problems with their nervous system.
To test the function of the visual system, the researchers performed electroretinograms to measure the electrical responses of photoreceptors, neurons that sense light, in the eyes at different times during the life of the adult fruit flies. They found progressive age-dependent loss of photoreceptors and their activity in the mutant flies.
Further electron microscopy studies showed that the photoreceptors of the mutant flies have lump-like membrane-enriched inclusions that are absent in normal cells. In addition, the researchers also found structural malformations in the majority of the mitochondria, often a characteristic feature of Parkinson’s disease, as well as dramatic enlargement of lysosomes, intracellular structures involved in recycling of membranes and other cellular components. Altogether, these results indicated that the iPLA2-VIA gene was important to maintain proper membrane structure and shape.
Surprisingly, the researchers found the levels of most phospholipids, except ceramides that were increased, remained unaffected in these mutants. This suggested that iPLA2-VIA (PLA2G6) gene plays a role in the pathway that produces ceramides.
When flies lacking the iPLA2-VIA gene were treated with two drugs that block ceramide synthesis, desipramine and myriocin, there was a reduction in the level of ceramides in the cells. Interestingly, mutant flies treated with the drugs also presented with less neurodegeneration compared to untreated mutants. Moreover, after treatment with these drugs flies were less sensitive to mechanical stress, had improved visual function and fewer alterations in the lysosomes.
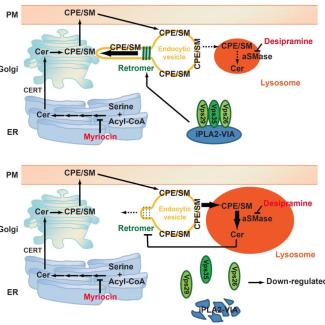
To investigate why ceramides were accumulating in the neurons of these mutant flies, the researchers probed further into how ceramide is synthesized and turned over in the neurons of these mutants. To maintain optimal balance of lipids in the membrane with the least expenditure of energy, cells reuse and recycle membrane lipids such as Ceramide Phosphoethanolamine (CPE) and Spingomyelin (SM) using intracellular recycling pathways.
Retromers are important components of the recycling pathway that identify CPE/SM, extract them before they reach the lysosomes and bring them back to membranes. CPE and SM that are not recycled are transported to the lysosomes where they are broken down into ceramides, which are then incorporated into the membranes of the cells.
The flies lacking the iPLA2-VIA gene had decreased levels of Vps35 and Vps26, proteins in retromers that are essential for their function. The researchers found that normally iPLA2-VIA protein binds to Vps35 and Vps26 and this binding enhanced retromer function.
In the absence of this binding, retromer function was disrupted due to which ceramide starts to accumulate in the membrane. Too much ceramide hardens the membrane, further disrupting retromer function and causing more CPE and SM to be shuttled to the lysosomes where they get hydrolyzed to produce more ceramides. This created a positive-feedback loop causing ceramides to accumulate, lysosomes to expand and disrupted neuronal function, leading to neurodegeneration.
Thus, this study has revealed a novel role for iPLA2-VIA protein in stabilizing retromer structure and its function.
They used various approaches to restore retromer function in fruit flies lacking the iPLA2-VIA gene and all of them resulted in significant improvement in the defects. These results were also confirmed in vertebrate neurons grown in the lab.
In addition, the researchers found that having high levels of alpha-synuclein, a hallmark of Parkinson’s disease, promoted retromer dysfunction, expanded lysosomes and led to accumulation of ceramide. Interestingly, these defects triggered by excess alpha-synuclein also were reduced by treatment with the same drugs that improved the defects observed in fruit flies lacking the iPLA2-VIA gene.
Altogether, this exciting work connects many new and previously identified players associated with the Parkinson’s disease puzzle, and has identified ceramide levels as a potential new therapeutic target for early onset Parkinsonism.